
U.S. Food and Drug Administration (FDA) Regulations
3
designated as the U.S. Agent for foreign facilities has agreed to
serve in that role. FDA will not provide the facility with a registration
number or confirm the registration renewal until the person confirms
they agree to the designation.
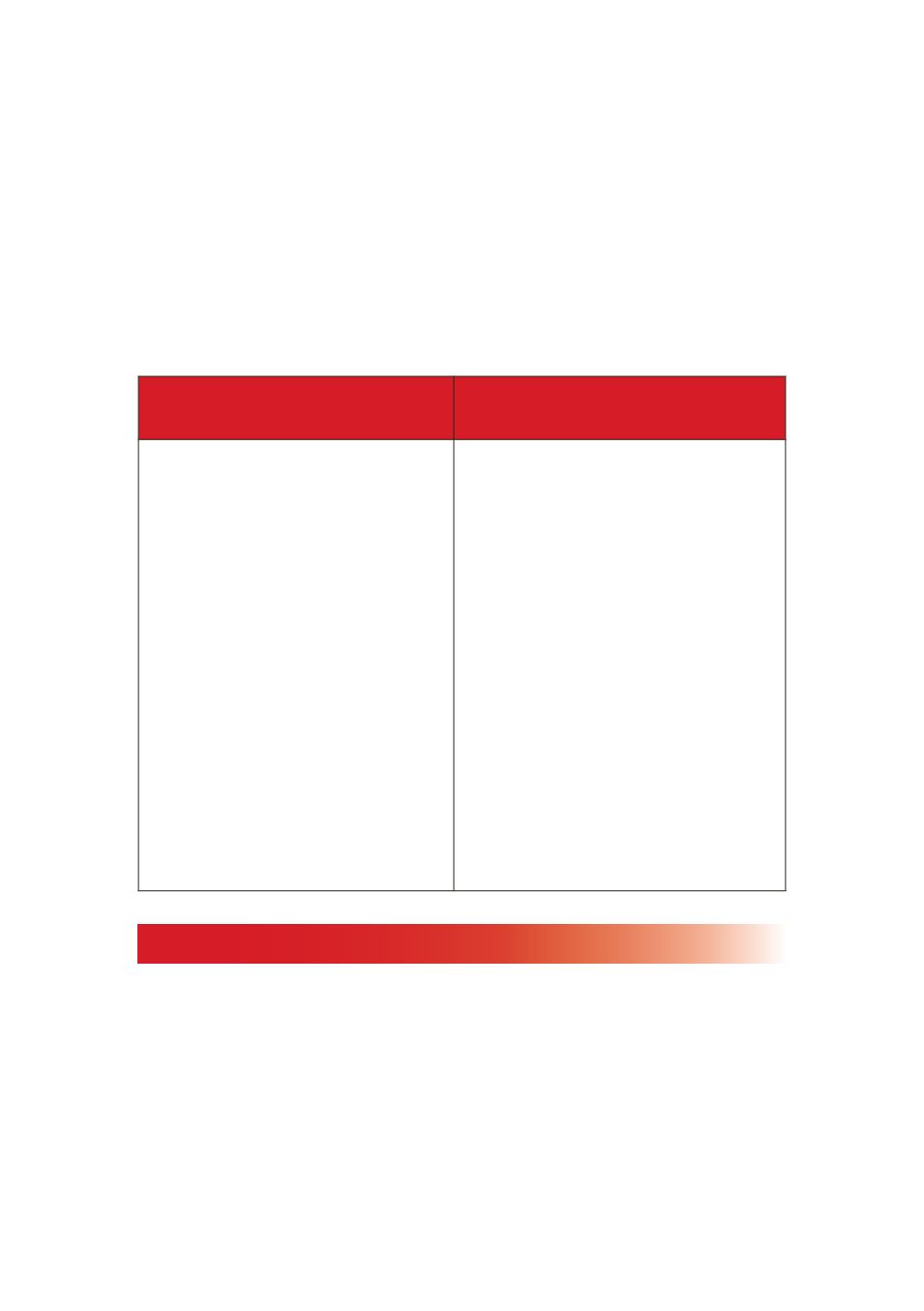
The facility of food product / activity categories has expanded as
follows:
Human Food Product
Categories and Activity Types
Animal Food Product Categories
•
Warehouse/Holding facility
will be divided into Ambient,
Refrigerated and Frozen.
•
Low Acid/Acidified food
processor will be divided
into Low Acid Food
Processor and Acidified
Food Processor
•
Farm “Mixed-Type” facility
will be added to the list of
activities.
•
Shellfish: Molluscan or
other.
•
New Product Categories
includes
botanicals
and
herbs; direct fed microbials;
forage products; and technical
additives.
•
Old categories replace with
new ones:
•
Animal derived products
Animal
protein
products
•
Food processing by-
products
Human food
by-products not otherwise
listed.
•
Recycled animal waste
products
Processed
animal waste products
FDA LABELING RULES
According to FDA Labeling Rules, there are changes to Food and
Dietary Supplement labeling.
Content Changes
•
Vitamin D and Potassium are now mandatory declarations
for the label.














